
| HOME |
ABOUT |
FACILITIES
EXPERTISE |
VANADIUM PROCESSING |
ZERO CARBON IRON MAKING |
TANTALUM TUNGSTEN |
VANADIUM ELECTROLYTE |
RARE
EARTH RECYCLING |
ELECTRODES |
ELECTROLYZERS |
PATENTS |
NEWS |
LINKS |
CONTACT |
 Français Français |
PROCESS III - RECYCLING RARE EARTH ELEMENTS FROM PHOSPHORS AND FLUORESCENT LAMPS
PROCESS II - EXTRACTING TANTALUM AND NIOBIUM FROM ORES AND CONCENTRATES
PROCESS VI - HIGH TEMPERATURE CHEMICAL PROCESS FOR THE PREPARATION OF CESIUM TUNGSTATEPCT of the International Patent Application WO 2023/060337 (A1) [PDF file (491 KB)] entitled HIGH TEMPERATURE CHEMICAL PROCESS FOR THE PREPARATION OF CESIUM TUNGSTATE (Inventor: François CARDARELLI) The present disclosure broadly relates to a high temperature chemical process for the synthesis of cesium tungstate in the solid state and the preparation of aqueous solutions or deuterated solutions of cesium tungstate. More specifically, but not exclusively, the present disclosure relates to a high temperature chemical process in which tungsten oxide compounds such as tungsten oxides, or natural or synthetic concentrates such as wolframite or scheelite, tungsten industrial by-products or their mixture thereof, are mixed with cesium compounds such as cesium carbonate, or cesium sulfate, or cesium hydroxide or their mixtures thereof and the mixture is roasted in air or oxygen at high temperature inside a kiln. After cooling, the solid sintered mass containing cesium tungstate is leached or dissolved with water or heavy water for producing dense aqueous solutions or deuterated solutions of cesium tungstate.US Patent Application 17/509,355 (April 13th, 2023) [PDF file (2.3 MB)]; Canadian Patent Application CA 3,140,407 A1 (April 11th, 2023) [PDF file (3.1 MB)]. |
||||||||||||||||||||||
 |
||||||||||||||||||||||
PROCESS V - ELECTROCHEMICAL PREPARATION OF VANADIUM ELECTROLYTES AND SULFATES OF MULTIVALENT TRANSITION METALSPCT of the International Patent Application WO 2022/213173 (A1) [PDF file (3.29 MB)] entitled ELECTROCHEMICAL PREPARATION OF VANADIUM ELECTROLYTES AND SULFATES OF MULTIVALENT TRANSITION METALS. (Inventor: François CARDARELLI) This novel electrochemical process addresses the preparation aqueous solutions of vanadium sulfates or aqueous solutions of transition metal sulfates and the recycling of end-of-life (E-o-L) vanadium electrolytes. More specifically, but not exclusively, the present disclosure relates to a direct electrochemical process in which a suspension, obtained by slurrying transition metals oxides such as oxides of vanadium, oxides of iron, oxides of cobalt, oxides of nickel, oxides of chromium, oxides of manganese, oxides of titanium, oxides of cerium, oxides of praseodymium, oxides of europium, oxides of terbium, oxides of uranium, oxides of plutonium, or their mixtures thereof with sulfuric acid as carrier fluid, is reduced electrochemically inside the cathode compartment of an electrolyzer to produce an aqueous solution of vanadium sulfates or of transition metal sulfates. Simultaneously, concentrated sulfuric acid and oxidizing co-products such as oxygen gas, peroxodisulfuric acid (H2S2O8), ammonium persulfate [(NH4)2S2O8], chromic acid (H2CrO4), cerium (IV) sulfate [Ce(SO4)2], manganese dioxide (MnO2), and vanadium pentoxide (V2O5) from spent vanadium electrolyte can be produced inside the anode compartment.US Patent Application 17/235,857 (October 20th, 2022) [PDF file (2.3 MB)]; Canadian Patent Application CA 3,117,596 A1 (October 10th, 2022) [PDF file (3.1 MB)]. |
||||||||||||||||||||||
 |
||||||||||||||||||||||
PROCES IV - CHEMICAL PROCESS FOR
RECOVERING VANADIUM AND IRON VALUES FROM VANADIFEROUS TITANOMAGNETITE -
VANADIUMCORP-ELECTROCHEM PROCESS TECHNOLOGY (VEPT) [PATENT SOLD TO VANADIUMCORP]
PCT of the
International Patent Application WO
2018/152628 (A1)
[PDF file (4.54 MB)]
entitled METALLURGICAL
AND CHEMICAL PROCESS FOR RECOVERING VANADIUM AND IRON VALUES FROM
VANADIFEROUS TITANOMAGNETITE AND VANADIFEROUS FEEDSTOCKS. (Inventor: François
CARDARELLI) This novel chemical process addresses the recovery of
vanadium, iron, titanium and silica values from a plethora of
vanadiferous feedstocks such as vanadiferous titanomagnetite, iron ores
and concentrates: magnetite and hematite, vanadium containing
industrial wastes such as BOF-slags and other industrial by-products
also containing vanadium. The process broadly comprises digesting the
vanadiferous feedstocks into concentrated sulfuric acid owing to the
exothermic sulfation reaction that allows to operate
quasi-autogeneously while producing a sulfation cake. The dissolution
of the sulfation cake after separating the insoluble solids
yields a concentrated pregnant solution. After reducing
electrochemically the pregnant solution, the reduced pregnant
solution is then subjeced to to chilling and crystallization to yield
clean crystals of copperas (ferrous sulfate heptahydrate: FeSO4.7H2O),
and produces an iron depleted solution. The process further comprises
removing titanium as titanium hydrolyzate [TiO(OH)2] by
hydrolysis from the iron depleted solution thereby producing a
vanadium-bearing pregnant solution. The further concentration by
evaporation and then a sequence of chilling and crystallization yields
vanadyl sulfate pentahydrate crystals [VOSO4.5H2O] to be
purified further to prepare various vanadium chemicals such
as vanadium pentoxide both to be used as precursors for the preparation
of vanadium
electrolyte (VE). |
||||||||||||||||||||||
 |
 |
|||||||||||||||||||||
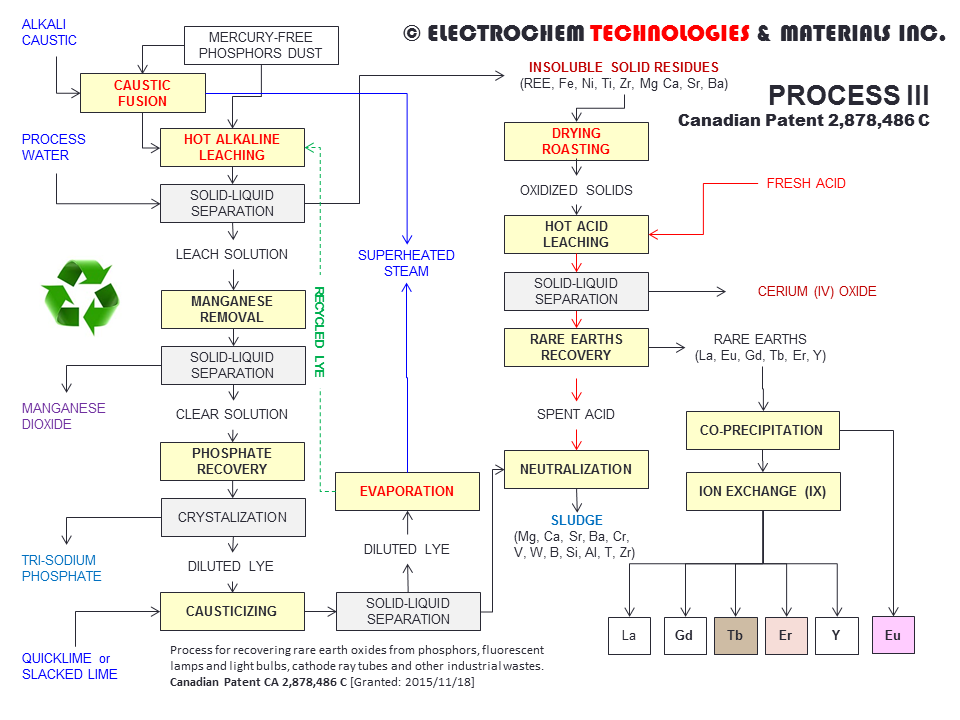
PROCESS III - RECYCLING RARE EARTH ELEMENTS FROM PHOSPHORS AND FLUORESCENT LAMPSPCT of the International Patent Application WO 2014/071510 (A1) [PDF file (3 MB)] entitled PROCESS FOR RECOVERING RARE EARTH OXIDES FROM PHOSPHORS, FLUORESCENT LAMPS AND LIGHT BULBS, CATHODE RAY TUBES AND OTHER INDUSTRIAL WASTES. (Inventor: Francois CARDARELLI) This novel process addresses the recovery of rare earths oxides from phosphor dusts, fluorescent lamps and light bulbs, cathode ray tubes and other industrial wastes containing rare earths elements in the form of halophosphates, tri-band phosphors and other fluorescent materials. The process consists: (1) to submit the wastes or spent materials to either caustic fusion or hot alkaline leaching; (2) to perform the hot alkaline leaching of the solidified melt to produce insoluble solid residues and a barren alkaline leaching solution; (3) To recover the tri-basic sodium phosphate by crystallization from the barren solution;(4) to oven-dry or calcine the insoluble solid residues; (5) to perform the hot acid leaching of the oven-dried or calcined solids; (6) to recover the cerium (IV) oxide from insoluble solids; (7) to reduce the cerium-free solution, precipitate, acid leach, precipitate, calcine and recover the europium (III) oxide; (8) to precipitate the remaining rare earths as oxalates; (9) to calcine the rare earths oxalates and dissolve the rare earths oxides in acid; (10) to separate the yttrium, gadolinium, terbium and erbium by ion exchange (IX) or solvent extraction (SX); (11) to precipitate and calcine the pure oxalates to yield pure yttrium (III) oxide, gadolinium (III) oxide, terbium (III, IV) oxides, and erbium (III) oxide; (12) Finally, to regenerate and recycle the spent alkaline solution after causticizing with calcium oxide or calcium hydroxide; Canadian Patent CA 2,878,486 C, February 9th, 2016 [PDF file (1.84 MB)]. |
||||||||||||||||||||||
 |
 |
|||||||||||||||||||||
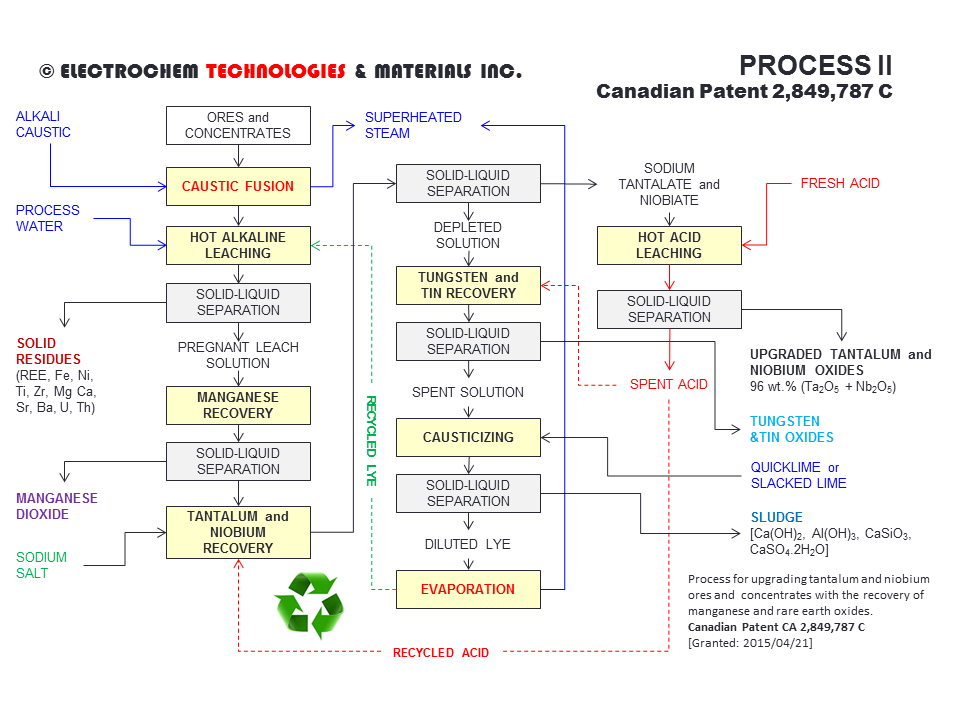
PROCESS II - EXTRACTING TANTALUM AND NIOBIUM FROM ORES AND CONCENTRATESPCT of the International Patent Application WO 2013/040694 (A1) [PDF file (3 MB)] entitled PROCESS FOR UPGRADING TANTALUM AND NIOBIUM ORES AND CONCENTRATES WITH THE RECOVERY OF MANGANESE AND RARE EARTH OXIDES. (Inventor: Francois CARDARELLI) - The process consists to upgrade tantalum and niobium ores and concentrates such as tantalite, pyrochlore in order to produce tantalum pentoxide (Ta2O5) and niobium pentoxide (Nb2O5). The chemical process comprises: (1) submitting the ground ore or concentrate to a caustic fusion or an alkali fusion using either KOH or NaOH to produce a solidified melt; (2) submitting the solidified melt to an alkaline leaching step to produce an alkaline pregnant leaching solution (PLS) comprising tantalum, niobium, and manganese values; (3) recovering the manganese values from the alkaline pregnant leaching solution either by electrolysis or by precipitation to produce potassium permangante (KMnO4) or manganese dioxide (MnO2); (4) precipitating tantalum and niobium from the manganese-free leach solution as insoluble sodium hexaniobiate (Na8Nb6O19) and sodium hexatantalate (Na8Ta6O19). (5) performing the hot acid leaching the sodium hexaniobiate and sodium hexatantalate to yield a mixture of pure niobium and tantalum oxides. Finally (6) The rare earth oxides are recovered from the insoluble solid residues obtained during alkaline leaching; Canadian Patent CA 2,849,787 C, April 21st, 2015 [PDF file (3.30 MB)]. |
||||||||||||||||||||||
 |
 |
|||||||||||||||||||||
PROCESS I - ELECTROWINNING IRON
AND REGENERATING SULFURIC ACID FROM COPPERAS AND FERROUS SULFATE
SOLUTIONS [ZERO-CARBON IRON
MAKING FerWIN™ PROCESS]
PCT
of the International Patent Application WO
2009/124393 (A1) [PDF
file (2 MB)] entitled ELECTROCHEMICAL
PROCESS
FOR THE RECOVERY OF METALLIC IRON AND SULFURIC ACID VALUES FROM
IRON-RICH SULFATE WASTES, MINING RESIDUES, AND PICKLING LIQUORS.
(Inventor: Francois
CARDARELLI) - The patent
describes a novel electrochemical technology called the FerWIN Process
for recovering metallic iron and iron-rich alloys and concurrently
regenerating sulfuric acid from iron-rich sulfate wastes, such as
ferrous sulfate heptahydrate (FeSO4.7H2O),
also
called copperas in the trade, currently by-produced from the titanium
white pigment industry, spent pickling liquors (SPLs) originating from
iron and steel making plants, and finally pregnant leach solutions
(PLS) generated during the acid leaching of ores and concentrates at
various minerals and metals processing plants. |
||||||||||||||||||||||
|
||||||||||||||||||||||
 |
 |
|||||||||||||||||||||
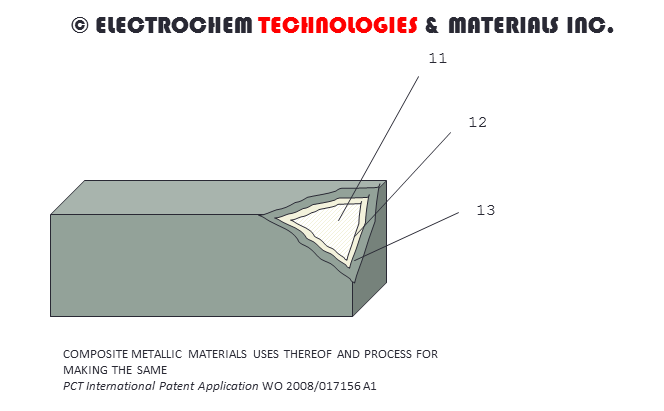
COMPOSITE METALLIC MATERIALSPCT International Patent Application WO 2008/017156 A1 [PDF file (3 MB)] entitled COMPOSITE METALLIC MATERIALS USES THEREOF AND PROCESS FOR MAKING THE SAME. (Inventor: Francois CARDARELLI) - A lightweight, high strength and corrosion resistant composite metallic material is disclosed herein. The composite metallic material typically comprises a high-to-weight ratio, low density core material; and a corrosion resistant protective refractory metal layer. The method for making the composite metallic material comprises the steps of surface activating the core material and forming a refractory metal on the surface of the surface activated core material by physical, chemical or electrochemical processes. Such a composite material is suitable for making biomaterials, corrosion resistant equipment and industrial electrodes. |
||||||||||||||||||||||
 |
 |
|||||||||||||||||||||
 |
Heavy Liquids for the Separation of
Minerals: Their Preparation, Properties, and Uses
Author:
François
Cardarelli
Publisher:
Electrochem Technologies & Materials Inc., Montreal
(QC), Canada
Pages :
xxiv, 537 pages, 285
tables, 76 figures B/W and Color
Format: 6 inches x 9 inches (15.24 cm x 22.86 cm) ISBN
978-1-7775769-6-7 Softcover
March 24, 2025
ISBN 978‐1‐7775769‐7‐4 eBook
March 24, 2025
This
monograph is primarily intended to describe the main physical
and
chemical properties of heavy organic liquids (e.g., bromoform,
tetrabromoethane, diiodomethane), dense aqueous
solutions of salts of inorganic acids
(e.g., halides, sulfates, nitrates, perchlorates, molybdates,
tungstates, metatungstates, polytungstates, polyoxometalates,
tantalates, perrhenates, etc.), and salts of organic acids (e.g,
formates, acetates, oxalates, malonates, etc.) and heavy suspensions
used in mineralogy, gemology, mining, metallurgy, and chemistry for the
separation by gravity of minerals, metallic ores,
graphite, coal
macerals, fossils, diamonds and gemstones along with plastics, glass,
ceramics
and
other synthetic materials. Moreover dense solutions of cesium and
rubidium salts used in
biology
for the separation of nucleic acids, and other biological molecules of
interest
by centrifugation using density gradients are also described. For several
heavy liquids, it provides a detailed description of the laboratory
methods,
and industrial processes utilized for their preparation along with the
most efficient
recovery and recycling techniques. Moreover, when available the
occupational
health and safety information for toxic and hazardous chemicals used
as heavy liquids is also provided to ensure safe practices in the work
place. Finally,
their potential utilization in others fields such as X-ray and
radiography
contrast agents, radiation shielding agents, non-destructive testing,
water-in-salt electrolytes for energy storage applications, oil drilling fluids, ballasts and
counterweights due to
their high density are described. The information has been presented in
such a
form that mineralogists, chemists, geologists, paleontologists,
biologists,
metallurgists, mineral processing engineers, scientists, professors,
and
technologists will have access to relevant scientific and technical
information
supported by key data gathered from several disseminated sources. The
following topics are covered:
www.amazon.ca www.amazon.de | www.amazon.se | amazon.com.br | amazon.com.au | www.amazon.co.jp Also available from these booksellers: Google Play | GoogleBooks |
 |
Electrowinning Iron and Recycling Sulfuric
Acid from Iron Sulfates: a Zero-Carbon Iron-Making Process
Author:
François Cardarelli
Publisher:
Electrochem Technologies & Materials Inc., Montreal
(QC), Canada
Pages :
xxviii, 471 pages, 181
tables, 140 figures B/W and Color
Format: 6 inches x 9 inches (15.24 cm x 22.86 cm) ISBN
978-1-7775769-3-6 Softcover
November 7, 2023
ISBN 978‐1‐7775769‐4‐3
eBook
November 6, 2023
This
comprehensive monograph is primarily intended to
describe the patented FerWIN® technology, a green and zero-carbon
iron-making
process, which consists to perform the electrowinning of iron metal and
the
recycling of sulfuric acid from iron sulfates that are by-produced at
the
million tons scale worldwide while releasing pure oxygen gas. The
information has been presented in such a form that
industrial electrochemists, chemical engineers, metallurgists, and
other
practicing engineers, scientists, professors, and technologists will
have
access to relevant scientific and technical information supported by
key
experimental data that were obtained from extensive laboratory,
prototype, and
pilot testing. It also includes comprehensive electrochemical and
engineering
calculations, costs and benefits analysis, financial and sensitivity
analysis. This
monograph will be of value also to men and women
engaged in the traditional iron and steelmaking industries that want to
understand this novel electrochemical technology outside their
conventional
blast furnace, direct reduced iron, and electric arc smelting processes. Finally,
the monograph may be of interest to persons
in the steelmaking industries occupying managerial positions such as
chief
executives, chief operating officers, and V.P. of operations. The
following topics are covered:
www.amazon.ca www.amazon.de | www.amazon.se | amazon.com.br | amazon.com.au | www.amazon.co.jp Also available from these booksellers: Google Play | GoogleBooks |
 |
Sulfuric Acid Digestion, Sulfuric Acid
Baking, and Sulfation Roasting in Mineral and Chemical Processing, and
Extractive Metallurgy
Author:
François Cardarelli
Publisher:
Electrochem Technologies & Materials Inc., Montreal
(QC), Canada
Pages :
xvi, 285 pages, 93
tables, 76 b/w figures
Format: 6 inches x 9 inches (15.24 cm x 22.86 cm) ISBN
978-1-7775769-0-5 Softcover
ISBN 978-1-7775769-2-9 Hardcover ISBN 978‐1‐7775769‐1‐2
eBook
This monograph is
primarily intended to serve as a concise review of the industrial
utilization of sulfuric acid and the plethora of sulfation techniques
used extensively in the mineral, chemical, and metallurgical industries
across the world.
The information has been presented in such a form that industrial chemists, chemical engineers, and other practicing engineers, scientists, professors, and technologists will have access to relevant scientific and technical information supported by key data gathered from several disseminated sources along with a brief description of each major industrial processes (e.g., phosphates, titanium dioxide, lithium, alumina, and beryllium), and finally several novel sulfation technologies that might be implemented in the near future. This monograph will be of value also to men and women engaged in other branches of chemistry and metallurgy that want to understand these techniques outside their field of expertise. Finally, the monograph may be of interest to persons in the chemical and metal industries occupying nontechnical positions such as executives, patent attorneys, traders, purchasing agents, salesmen and women, to whom a general knowledge of the technical aspect of their business would be helpful The following topics are covered: • Physical and chemical properties of sulfuric acid and oleums; • Corrosion resistant materials; • Thermochemistry of sulfation reactions; • Industrial sulfation processes; • Novel sulfation processes; • By-products, effluents, and wastes; • Concentration and regeneration of sulfuric acid; • Prototype and pilot testing; • Health and safety; • Economic data; • Appendices. For purchase visit amazon bookstores: www.amazon.ca www.amazon.de | www.amazon.se | amazon.com.br | amazon.com.au | www.amazon.co.jp Also available from these booksellers: Google Play | GoogleBooks |
 |
MATERIALS
HANDBOOK. A Concise Desktop Reference. THIRD EDITION
by Francois CARDARELLI SPRINGER International Publishing, London CXXXVIII, 2254 pages and 173 black and white figures, 2 illustrations in color (2018) in two volumes ISBN 978-3-319-38923-3 Hardcover ISBN 978-3-319-38925-7 eBook ISBN 978-3-030-13247-7 Softcover https://doi.org/10.1007/978-3-319-38925-7 Library of Congress Cataloging Number: 2018940467 The unique and practical Materials Handbook (third edition) provides quick and easy access to the physical and chemical properties of very many classes of materials. Its coverage has been expanded to include whole new families of materials such as minor metals, ferroalloys, nuclear materials, food, natural oils, fats, resins, and waxes. Many of the existing families - notably the metals, gases, liquids, minerals, rocks, soils, polymers, and fuels - are broadened and refined with new material and up-to-date information. Several of the larger tables of data are expanded and new ones added. Particular emphasis is placed on the properties of common industrial materials in each class. After a chapter introducing some general properties of materials, each of twenty classes of materials receives attention in its own chapter. The health and safety issues connected with the use and handling of industrial materials are included. Detailed appendices provide additional information on subjects as diverse as astronomical data, crystallography, spectroscopy, thermochemical data, analytical chemistry, corrosion resistance, and economic data for industrial and hazardous materials. Specific further reading sections and a general bibliography round out this comprehensive guide. The index and tabular format of the book makes light work of extracting what the reader needs to know from the wealth of factual information within these covers. François Cardarelli has spent many years compiling and editing materials data. His professional expertise and experience combine to make this handbook an indispensable reference tool for scientists, chemists, geologists and engineers working in numerous fields ranging from chemical to nuclear engineering. Particular emphasis is placed on the properties of common industrial materials in each class. After a chapter introducing some general properties of materials, materials are classified as follows: CONTENT: Front Matter and Table of Content (ToC) [PDF file (1.39 MB)] - Properties of Materials.- Ferrous Metals and Their Alloys (Pig iron and steels. - Nickel and nickel alloys. - Cobalt and cobalt alloys. - Manganese and manganese alloys). - Ferroalloys. - Common Nonferrous Metals (Aluminum and aluminum alloys. - Copper and copper alloys. - Zinc and zinc alloys. - Lead and lead alloys - Tin and tin alloys). - Minor Metals (Cadmium. - Mercury. - Gallium. - Indium. - Thallium. - Arsenic. - Antimony. - Bismuth. - Selenium. - Tellurium.) - Less Common Nonferrous Metals (Alkali-metals, Alkaline-earth metals). - Refractory and reactive metals (Titanium and titanium alloys, Zirconium and zirconium alloys, Hafnium and hafnium alloys, Vanadium and vanadium alloys, Niobium and niobium alloys, Tantalum and tantalum alloys, Chromium and chromium alloys, Molybdenum and molybdenum alloys, Tungsten and tungsten alloys, Rhenium and rhenium alloys) - Noble and precious metals (Silver and Gold). - Platinum Group Metals (PGMs, Ruthenium, Rhodium, Palladium, Osmium, Iridium, Platinum). - Rare Earths and Lanthanides. - Actinides (Uranium, Thorium and Plutonium) .- Semiconductors.- Superconductors.- Magnetic Materials.- Insulators and Dielectrics.- Miscellaneous Electrical Materials [PDF file (1.85 MB)]. - Ceramics, Refractories and Glasses.- Polymers and Elastomers.- Minerals, Ores and Gemstones.- Rocks and Meteorites.- Soils and Fertilizers.- Cements, Concrete, Building Stones and Construction Materials.- Timbers and Woods.- Fuels, Propellants and Explosives.- Nuclear Materials. - Composite Materials.- Gases.- Liquids. - Foods, Oils, Resins & Waxes. - Materials Occupational Health & Safety. - Appendices. - Bibliography. - Index. [PDF file (6.25 MB)]. For purchase see: www.springer.com or any bookstore such as www.amazon.ca |

|
ENCYCLOPAEDIA
of SCIENTIFIC UNITS, WEIGHTS and MEASURES.
Their SI equivalences and origins.
by Francois CARDARELLI SPRINGER London, New York, Heidelberg xxiv, 848 pages (2005) ISBN 1-85233-682-X Hardcover. ISBN 1-60119-016-6 eBook https://doi.org/10.1007/978-1-4471-0003-4 Library of Congress Cataloging No. QC94.C295 2003 With more than 5,000 units and 35,000 conversion factors The Encyclopaedia of Scientific Units, Weights and Measures covers the full gamut of science, technology and medicine, and deals with US customary, Imperial British, conventional metric, MTS, MKpS, MKSA, CGS (esu, emu and Gaussian), FPS, IPS, atomic units (au), historic and SI units. The book allows to convert the huge variety of units from all over the world in every period of recorded history into units of the SI. Featuring: * An A - Z of conversion tables for over 5,000 units of measurements. * Tables of units arranged according to the physical quantities (e.g., mass, length, area, volume, density, viscosity, etc.). * Tables of mathematical and fundamental constants. * Listings of professional societies, and national standardization bodies for easy reference. * An extensive bibliography detailing further reading on the multifarious aspects of measurement and its units. This huge work is simply a "must have" for any reference library frequented by scientists, engineers, and students of any discipline or by those with historical interests in units of measurement such as historians and archaeologists. CONTENTS [Detailed Table of Content & List of Tables PDF file (88 KB)]: - Introduction.- The International System of Units (SI). - Other Systems (UK, US, FPS, MTS, MKpS, CGS, IEUS, atomic units, ancient times systems, and obsolete national systems). - Conversion Tables. - Mathematical and Fundamental Constants. - Appendices. - Bibliography. For purchase see: www.springer.com or any bookstore such as www.amazon.ca |
 |
 |
 |
| [1]
ELECTROWINNING OF METALLIC IRON FROM IRON SULFATE(S) [PDF file (7.4 MB)] |
[2]
VANADIUM ELECTROLYTE PRODUCTION AND RECYCLING [PDF file (6.7 MB)] |
[3] MIXED METAL OXIDES (MMO) ANODES [PDF file (4.5 MB)] |
 |
 |
 |
| [4]
VANADIUMCORP-ELECTROCHEM PROCESS TECHNOLOGY (VEPT) [PDF file (6.7 MB)] |
[5] CESIUM
TUNGSTATE FOR HEAVY MINERALS SEPARATION [PDF file (7.4 MB)] |
[6] RECYCLING OF RARE EARTH from FLUORESCENT
LAMPS [PDF file (5.5 MB)] |